Vidéo trouvée sur Points de Vue
Vidéo trouvée sur Points de Vue
Article trouvé sur : www.pointsdevue.com
Light-induced ocular damage has been investigated for decades in laboratory extensive work and several epidemiological studies. More recently, harmful effects of blue-violet light have been spotlighted by growing body of scientific research. Despite the eye’s natural defense mechanisms, it has been evidenced that cumulative exposure to blue-violet light can contribute to long-term irreversible changes in the retina. When the most critical exposure occurs in outdoor conditions, Transitions® lenses can effectively filter harmful blue-violet light and consequently provide optimal photo-protection for the patient eyes.
Light is an element of life, a major environmental factor in human development. It plays a significant role in how we process sensory information, impacting our visual experience from the point of birth and throughout our lives.
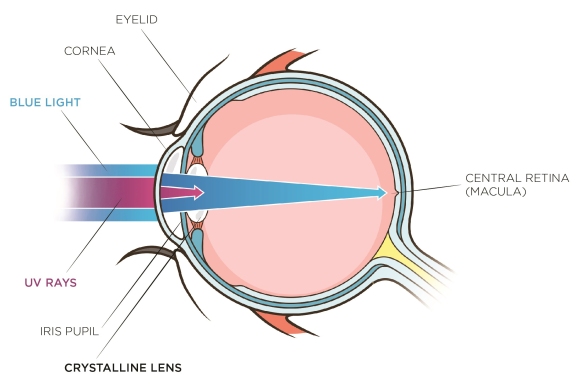
Visual perception occurs when light strikes the retina of the eye. The pupil of the iris serves as the optical diaphragm of the eye affecting the path of light rays which are refracted by the cornea and the crystalline lens on their way to the retina. Numerous deprivation experiments have demonstrated that ocular growth and refraction development are regulated by visual information. Light is essential in providing this information on diurnal species by transmitting signals which are converted by the brain into visual perception. This acquisition of visual function is experienced as early as infancy and is essential to healthy development.
The iris acts as a natural optical diaphragm for expanding (dilation) or retracting (constriction) its central aperture. Depending essentially on lighting conditions and age, the diameter of the pupil ranges from 2mm to 8mm. Variations in the diameter of the pupil are caused by a movement reflex that regulates the light flux incident and, subsequently, visual performance. The visual system as a whole is sensitive over a wide range of light levels from starlight to bright sunlight but, despite the regulation of the pupil aperture, it cannot operate over the entire range simultaneously. An adaptation is required to adjust the light sensitivity of the visual system to different light levels. When the adaptation is in progress, visual performance is reduced. Once the process is complete, visual capabilities depend on the new level of light.
There are two primary lighting conditions with which the visual system has to deal: daylight (photopic) and nighttime (scotopic). Between photopic and scotopic levels is a range called mesopic, which corresponds roughly to twilight. The human eye has three types of light sensitive cells (photoreceptors) in the retina – cones, rods and ganglion cells – that process sensory information (Table 1). Cones are highly concentrated in the central area of the retina (macula) and are responsible for providing daylight sharp image resolution and color detection. Rods are largely distributed in the periphery of the retina. Having high sensitivity, they are required for scotopic vision but provide low resolution and lack of color information. The ganglion cells or ipRGCs (intrinsic photosensitive Retinal Ganglion Cells) express the melanopsin-based photopigment. These melanopsin ganglion cells are crucial for relaying light information from the retina to the brain to control circadian rhythms, pupillary light reflex, sleep and many other body functions. (Sand A. et al., 2012, Gronfier 2013).[11, 09]

Table 1. Summary of main lighting conditions (Boyce, 2001).[6]
The sun emits a tremendous amount of energy in the form of wide electromagnetic radiation. From cosmic rays to radio waves (Fig. 1), the majority of solar emissions are not visible to human photoreceptors. Only a thin portion – at wavelengths (λ) between 380nm and 780nm – provides the visible light that interacts with the eye’s photoreceptors – enabling us to see the world. When visible solar radiation reaches the Earth’s surface it is scattered throughout the atmosphere, especially in the blue-violet region corresponding to the shortest wavelengths (380-460nm) of visible light and subsequently to the highest energy.

Fig. 1: Electromagnetic radiation and the visible spectrum
Beyond the visible spectrum, sunlight emits ultraviolet radiation with wavelengths shorter than 380nm – commonly referred to as UV – and infrared radiations with wavelengths greater than 780nm. Ultraviolet radiation arriving on earth surface is divided into UVB (280-315nm) and UVA (315-380nm). At sea level, about 10 percent of radiation is UV, 50 percent is visible and 40 percent is infrared.
Exposure to the sun for an extended period of time produces erythema and affects skin pigmentation, causing burning or tanning. Both UVA and UVB penetrate the atmosphere freely and play a critical role in advancing more severe health conditions like premature skin aging (ex: wrinkles) and certain skin cancers (ex: carcinoma) which can affect the eyelids and facial skin. In a healthy adult, more than 99 percent of UV radiation is absorbed by the anterior part of the eye (eyelid, ocular surface, crystalline lens). Exposure to ultraviolet radiation is well established as a major cause of eyelid malignancies, photokeratis, climatic droplet keratopathy, pterygium and cortical cataract (Yam 2014, Behar-Cohen et al. 2014). [17, 3] There is insufficient evidence to support the proposal that Age-related Macular Degeneration (AMD) is related to UV exposure, and it is now suggested that AMD risk is probably more closely related to exposure to visible radiation, especially blue light (Yam 2014). [17]
The blue sky is evidence that blue light is present in direct sunlight. Since blue light is higher in energy than other wavelengths in the visible spectrum (Fig. 2), it scatters more throughout the atmosphere (Rayleigh scattering) and makes the sky appear blue. Blue light makes up 25-30 percent of daylight.

Fig. 2: Daylight source spectra
While blue light is emitted naturally by the sun, it can also be produced by numerous artificial light sources commonly found indoors. Light-emitting diodes (LEDs) are gaining an increased share of the domestic lighting market because of their high efficiency of luminance and low energy consumption. Widely found in digital screen technologies and displays, LEDs exhibit a high emission blue peak, centered at 430nm (Fig. 3).

Fig. 3: Artificial cool white LED source spectrum
As a part of visible light, blue light passes through the eye structure, reaching the retina. Due to its higher level of energy than the other wavelengths in the visible spectrum, it is potentially harmful to the retina. Depending on exposure conditions (light intensity, duration, periodicity) it may induce different types of reactions, including photochemical lesions (Rozanowska et al., 2009). [16]. Laboratory experiments showed that blue light is harmful (Sparrow et al., 2000)[14] and particularly it has been demonstrated that exposure to blue violet light with a maximum peak centered on 435+/- 20 nm can induce irreversible cell death in the retinal pigment epithelium (RPE), located in the external layer of the retina (Arnault et al., 2013). [1] These damages contribute to the aging process of the eye and may lead to the development of pathologies such as AMD, the major cause of blindness in the elderly in developed countries. In epidemiological studies addressing long term chronic exposure to blue light, the Beaver Dam Eye study demonstrated that there is a strong correlation between outdoor activities (sunlight exposure) and early incidence of AMD changes (Cruickshanks et al., 2001, Tomany et al., 2004). [7, 15]
Amount of blue violet light is characterized by the intensity of emitted light of varied sources (Table 2). Sunlight is by far the strongest source of blue light at least 100 times greater than artificial sources (Fig. 4).

Table 2: 420-440 nm integrated Irradiance values (w/m2) of common artificial light sources against solar diffused light (Transitions Optical internal measurements)
There is a significant difference in the level of blue light when facing into the sun (direct) and facing away from the sun (indirect). In actuality, no one looks directly at the sun since there is a natural aversion to sources of high glare. Humans often make adjustments by moving their head or their eyes or by relying on automatic reflexes like blinking, squinting and pupillary constriction. The eye can be subject to more serious effects due to multiple reflections of sunlight onto white surfaces. For example, the reflection of the sun at noon on sand or snow can reach 10 times more luminance than the blue sky (Behar-Cohen et al., 2011). [4]
The impact of blue-violet light exposure depends on the amount of total light reaching the retina: the retinal irradiance, which is characterized by the radiant flux (power) received by the retina per unit area. These values vary by the ocular media transmittance and – more importantly – by physical factors such as the eyelid position, which dictates the field of vision and the pupillary aperture, making ocular dosimetry far more complex than generally appreciated (Sliney 2001, 2005). [12, 13]More investigations need to be done, but it seems reasonable to think that the level of retinal irradiance in the 435+/- 20 nm range is more important outdoors than indoors. Wearing appropriate glasses can be worthwhile to prevent from cumulative effects of light exposure.


Fig. 4: Irradiance spectra of common artificial light sources (top) and direct and indirect sunlight (bottom). (Transitions Optical internal measurements)
Physiological structures around the eye, like eyelids and eyelashes, provide some protection against intense light. The iris pupil also contributes by using constriction to decrease the amount of entering light. While UV transmittance is blocked primarily by the cornea and crystalline lens in healthy adults, blue light crosses over these structures to reach the fundus of the eye (Fig. 5). The amount of blue light reaching the retina depends on the age of the eye as, during a lifetime, there is a yellowing of the crystalline lens that would typically provide some absorption in the blue violet region. The central part of the retina is covered by yellow pigments (Macula Lutea), which serve as a filter for incoming blue light because its absorbance peak in this range (Haddad et all, 2006).[10] Due to assorted factors, macular pigment density can be variable from one individual to another and its ability to absorb light evolves during a lifetime. The children are the most exposed to harmful blue light because they have larger pupil diameter, less concentration of macular pigment and the amount of blue light reaching the retina is 65 % while it is 40 % for adults (Behar-Cohen et al., 2015). [5]

Fig. 5: UV and blue-violet light path into the human eye
With the potential risks associated with outdoor conditions described and the natural protections of the human eye discussed, we now turn our attention to the technical solutions available within the eyewear industry to prevent from the long-term effects of blue-violet light. UV protection in eyewear will not be reviewed here since most high-quality lenses today offer complete protection against UV up to 380nm.
Anti-reflective interferential layers may be applied to ophthalmic lenses by evaporating transparent dielectric metal oxides to the anti-scratch coating on both the convex and concave sides of the lens. The coatings essentially involve stacks created by successive deposits. Processed under vacuum on a few hundred nanometers of low index material (RI ~1.46) and high index material (RI ~ 2.2) of desired thickness (Fig. 6), they provide anti-reflective properties within the visible region of the light spectrum. It is possible to design anti-reflective stacks that offer enhanced protection in the blue-violet light region by adding a specific reflection element at the wavelength to be rejected, in this case 380-460nm. The blue-filtering reflective properties can be effective up to 20 percent while keeping superior anti-reflective properties active within the entire remaining visible range. These ophthalmic lenses display high clarity indoors and outdoors, and offer reliable indoor protection against harmful blue-violet light emitted by electronic devices and artificial lighting while providing moderate outdoor protection as well.


Fig. 6: left: Blue mirror effect of an anti-reflective coating (AR) and its reflectance spectra (right)
Another way to prevent harmful blue-violet light from entering the eye is to reduce the unwanted wavelengths by absorbing them with yellow dye, a chemical compound whose structure allows absorption in the visible part of the light spectrum of its complementary color: in this case, blue. This is why most blue-absorbing lenses appear more or less yellow depending on the level of their blue-filtering properties. A highly-efficient blue-blocking lens would appear deep yellow, while a moderately efficient blue-blocking lens would appear merely yellowish.
The advantage of the yellow dye solution is that it can reduce a significant amount of blue light, but the intense yellow color is detrimental to its cosmetic appearance and detracts from human color perception. A highly intense yellow filter, for example, will induce color distortion despite the ability of the brain to adapt chromatically.
There is a way to circumvent the yellow color of an absorbing filter that involves “color balancing” the tint by adding a small proportion of another dye. The complementary dye absorbs in another region of the visible spectrum, creating a global neutral grey filter (Fig. 7). This solution is acceptable for low yellow colors – where color balancing can be efficient – but not possible for dark yellow tones. It should be noted as well that color balancing in general is detrimental to the global photopic transmission of a lens since it causes a loss of visible transmission (or clarity).
A lens can also be surface tinted by dipping an uncoated lens substrate or a tintable coated lens in a water dye solution at an elevated temperature.
Another solution is to cast lenses with monomers that already contain yellow dyes – and its color balancing agents – in the original formulation. In this case, only light tints are achievable since darker tints would lead to a non-homogeneous appearance from center to edge due to differences in prescription lens thickness (high-minus and high-plus finished lenses).

Fig. 7: Blue light absorption with yellow dyes in substrate (left) and neutral color-balanced substrate (right)
Sunwear lenses are commonly grouped by IS0 8983-3 standards as class 3, providing 10-15% of photopic transmission (Tv), or the darker class 4 category (Tv < 8%).
In the case of prescription eyewear, sun lenses are essentially made by diffusing a mixture of dyes in a polymer substrate or in a tintable coating. For the plano sunwear business, coloring is achieved by mass mixing an injection mold of polycarbonate for instance. Polarized lenses are made by using dichroic dyes in pre-formed stretched films or encapsulated wafers. The dyes are generally a mixture of primary colors in different combinations to achieve the desired hues based on the principle of subtractive color mixing (Baillet et al., 2008). [2] The most common hues are brown and grey.
In the fashion and high-performance sunwear business, one finds mirrored lenses manufactured on the principle of interferential light rejection stacks and/or a mix of tinting by absorption and rejection mirror technologies.
By definition and usage, sun lenses are made exclusively for outdoor purposes. The dark intensity of the lenses, both plano and Rx, allows very good protection against blue light, especially by brown lenses where the yellow dye content in the mixture is in the majority (Fig. 8).

Fig. 8: Sun lenses in brown and grey showing that, at equal photopic transmission (15% Tv), the brown lens filters more blue light than the grey lens, as it contains more yellow dyes in its formulation
Photochromic lenses are non-permanent tinted filters containing photochromic dyes made from molecular structures that are reversible under the action of light (DÜrr et al., 1990). [8] Their tint or color is obtained through the same principle of color-subtractive mixing as sunwear lenses.
There are, however, several notable differences in manufacturing technologies, including the cast in place (CIP) process wherein photochromic dyes are added to the monomers before polymerization, and the imbibition process, where photochromic dyes are absorbed into the surface of a lens. In these first two examples, a dedicated polymer allows the photochromic mechanism and movements to occur, and requires different polymers for each refractive index (for prescription lenses). The coating technology, meanwhile, wherein photochromic dyes are added to a coating deposited by dip – or preferentially, by spin – allows the process to be substrate independent. Photochromic lenses are highly efficient in protecting against glare, since their darkness (photopic transmission) automatically adjusts to the amount of outdoor light, whether overcast, in shadow or in bright sunlight. Because they always acclimate to various lighting levels, they help the visual system to adapt instantaneously without compromising visual performance or comfort.
The advantage of photochromic lenses like Transitions® Signature™ lenses is that they are dark outside when sunlight is bright and intense, so they offer a high level of blue light filtering much like regular sun lenses. They can be worn all the times and offer good indoor protection against artificial blue lights with no aesthetic drawbacks such as residual yellow color (Fig. 9).
As described before, color-balancing can help to limit the yellowish aspect of a given filter. For photochromic lenses, where a very low level of yellowness needs to be overcome, the smart color balancing is put to full use. Only a slight amount of dyes are used to deceive the eye (and subsequently the brain) to offset the yellowish aspect induced by chemical species providing the blue blocking properties.
A specific family of high technology products like Transitions® XTRActive® lenses, which allow activation of the photochromic molecules behind the windshield of a vehicle, present the unique advantage of having a light tint indoor and a strong tint outdoor, leading to enhanced blue light-filtering at all times (Fig. 9 and 10) thanks to specific proprietary photochromic molecules that intrinsically absorb in the blue region of the visible spectrum.
Fig. 9: Overlay of un-activated and activated spectra of Transitions® Signature™ grey and brown lenses [A] and Transitions® XTRActive® grey and brown lenses [B]

Fig. 10: Blue filtering protection offered by Transitions® lenses at 23°C (ISO 8980-3 calculation 380nm-460 nm range)
Visible light reaching the retina is essential for visual perception. Despite several self-protection mechanisms, the retina in the human eye can be exposed to light levels that exceed its natural defenses and can cause long-term irreversible damage. The lifelong buildup of light-induced phototoxicity can contribute to age-related changes and retinal cell degeneration.
Preventing excess exposure and accumulation of blue-violet light indoors – and especially outdoors – during one’s life seems like common sense.
Transitions® photochromic lenses – and, in particular, Transitions® XTRActive® lenses – offer the optimum visual experience, regardless of lighting conditions, while providing an ideal protection against blue-violet light under all circumstances (Fig. 11).

Fig. 11: Blue light benefits delivered by different optical solutions in the eyewear industry
Article trouvé sur : www.pointsdevue.com
Reference : Sheedy, J., Visual fatigue, Points de Vue, International Review of Ophthalmic Optics, N70
Many of our patients have symptoms of discomfort associated with performing near tasks. Of course, the most common tasks performed at near involve reading – especially at computer displays [1] . Therefore it becomes the task of the practitioner to diagnose the conditions causing the symptoms and to devise a treatment plan to eliminate or at least mitigate the symptoms.
The reading task itself, whether on hard copy or electronic display, is perhaps the most visually-demanding near task. Typical reading involves a series of 200 ms fixations sandwiched between 35 ms saccades – each saccade moves the eyes 7-9 characters further in the text. Although this is very demanding, we have discovered that it is the cognitive uptake system that limits reading speed in subjects with vision systems that are performing well – not the visual system. By manipulating the text size and legibility we have noted that fixation durations and frequency are altered, but the reading speed is maintained [2, 3, 4, 5] . Actually, it is remarkable that many people can read for hours with no symptoms.
Given that reading (near work) can be performed without symptoms under good conditions, it becomes our task to identify the reason(s) why our particular patient has symptoms of discomfort. I have observed clinically [6] and in laboratory research [7, 8,9] that symptoms may occur when either the environmental conditions or the visual system capabilities are compromised. Resolving the patient symptoms often requires analyzing both the visual system and the environmental conditions under which they have the symptoms of discomfort [10].
Analysis begins with scrutiny of the patient symptoms. This can often directly lead the clinician to the correct diagnosis. The symptoms can be categorized into visual, musculoskeletal, and asthenopia as shown in Table 1.

Tab. 1: Three primary symptom categories.
Visual symptoms are the easiest to diagnose. They can easily result from an uncorrected refractive error – errors as low as 0.50 DC can result in symptoms.
Presbyopic patients should be properly corrected for the unique viewing distance of their computer, and will report blur or musculoskeletal ache if not. Typically presbyopic patients require an intermediate prescription in order to see their computer correctly. It is important to determine the distance at which they view their work (office computer displays are typically at a viewing distance of 50-60 cm). Demonstration and refinement of the near addition in free space can reassure both clinician and patient.
Slowness in focusing, or distance blur after near work, is typically due to accommodative infacility. If so, tests of accommodative function can assess if this is a problem. It is best to test accommodative infacility directly using +/- lens flippers.
Double vision (diplopia) is infrequently reported, but indicates a binocular vision difficulty when present. An intermittent diplopia usually indicates an intermittent strabismus. Analyze the binocular vision system to determine if there is an eso or exo strain on the visual system. The most common problem is a convergence insufficiency that causes intermittent exotropia at near distances.
Musculoskeletal sysmptoms
Neck ache and back ache are quite common in computer-using patients. This can often be due to inappropriate location of the display or inappropriate spectacle correction of presbyopia.
The top of the display should be near eye level. If not, then adjustments need made to accomplish this. Our visual system has a strong preference for looking down about 10 degrees – i.e. depressing the eyes about 10 degrees [11]. If the display center is not about 10 degrees below the eyes, then neck and back adjustments are made [12] resulting in strain.
Neck and backache can also be caused by presbyopia-correcting spectacles that cause an awkward viewing distance or posture to see the computer display or other uniquely-located near work. It is very common for general-issue bifocals or progressive addition lenses (even though they might work for most other everyday tasks) to be guilty of this.
Wrist, back, and shoulder pain or ache can also be caused by other work-related factors and referral to a workplace specialist is indicated.
Asthenopia is a catch-all for the less-specific symptoms such as eyestrain.
Our research has repeatedly shown [13–14] that these symptoms fall into 2 constellations both subjectively (i.e. patient sensations) and objectively (i.e. the inducing condition). We call these 2 constellations “external symptoms” and “internal symptoms”. They are summarized in Table 2.

Tab. 2: External and internal symptoms
In general, the differentiation can be summarized as follows:External and internal symptoms
The clinician can use this symptom differentiation to help guide the diagnosis and management of the patient. External symptoms indicate a dry eye condition and possible environmental culprits such as lighting, display location, or text quality. Internal symptoms indicate an ophthalmic or visual problem related to accommodation, convergence, or refractive error. Clinicians may want to use the clinical tests shown in Table 3 to diagnose accommodative and binocular vision disorders.

Tab. 3: Tests for accomodation and binocular alignment
After diagnosing the reasons, either environmental or visual, that cause or contribute to the symptoms of discomfort, then the appropriate treatment measures from those below can be used to treat the patient.
To begin, the location of the primary work (e.g. computer display) must be determined. If a computer display location can be altered, then it should be located so that the top of the display is level with the eyes. If the display cannot be located differently, then its location should be noted and spectacles designed accordingly.
Most younger presbyopes (near add of 1.25 D or less) can often use their regular bifocals or PAL for their intermediate work (e.g. computer display). This is because such patients have enough remaining accommodation that they are able to comfortably view and focus upon the intermediate task through the distant portion of their spectacle lenses.
Presbyopic patients with a near add of 1.50 D or greater often require separate spectacles for performing near work comfortably if that near work is at a unique viewing angle or distance, such as commonly occurs at computers or on assembly lines. If the patient wears bifocals for everyday needs, then it is likely best to provide the patient with work-related bifocals in which the top contains the intermediate prescription and the bottom contains the near prescription. Trifocals may be considered. If the patient wears PALs for everyday viewing, then it is best to provide Occupational Progressive Lenses (OPL) for the patient. OPLs are designed to provide extensive intermediate and near viewing areas. Usually the top of the lens also contains a small add of +0.50-0.75D. OPLs are very useful for most office and other indoor activities.
In prescribing adds and designing spectacles, it can be very useful to demonstrate the add and clear viewing distances in free space. If prescribing an OPL, it is also very useful to demonstrate the small distance blur through the top of the OPL so that there are no surprises at time of dispensing.
Dry eye is a common complaint among office and computer workers. Very often the following conditions contribute to dry eyes and fixing them can improve the symptoms:
In addition to the above measures, it is advisable to provide artificial tears to be used only as needed. Counseling about work breaks and light rubbing of the lids may also be helpful. More severe cases of dry eye require additional measures such as punctual plugs.
Reduced amplitude of accommodation (for the patient’s age) and accommodative infacility can both be managed with either orthoptic training or prescription of plus lenses (usually +0.50 to 1.00D) for near work. Working patients often are unwilling to spend the time with an orthoptic program, and the plus lenses can cure the problem.
Likewise, patients with esophoria at near are best treated with a near add, which reduces the eso stress on their binocular system.
Patients with exo deviation, as often accompanied by convergence insufficiency, must be treated with orthoptics – lenses are not an effective treatment. Fortunately, convergence is the most easily trained visual function and can often be managed with push-up training alone.
Lighting is likely the most common environmental culprit insofar as causing and contributing to visual discomfort. All patients with near viewing symptoms should be counseled about eliminating glare from lights.

The most common lighting problem is shown in the picture above: light from luminaires (or windows) directly impinging the eyes of the patient – i.e. the light source is very bright in the peripheral field of the patient. This can be demonstrated to the patient by taking the patient to an office location with a bright overhead light, and requesting the patient to shield their eyes from the offending light with their hand. Patients should be encouraged to note the improved comfort by doing so. The patient can then be instructed to repeat the test at their work place to test if lighting is a problem.
If lighting is determined to be a problem, then possible interventions include: turning off the offending light, use blinds or drapes on windows, remove white surfaces, use partitions, rotate the work station, use indirect lighting, or wear a visor.
For visual and musculoskeletal comfort, the work to be viewed most often must be directly in front of the person and located so that the person views it with eyes depressed at least 10 degrees and no more than 30 degrees. For computer displays, intended to be used with an upright posture, the top of the display should be at eye level, resulting in eye depression to view all elements of the display.
Upright posture while maintaining the normal convex curvature of the lower spine can be important to long term comfort. Arms should be supported by chair arm rests to avoid tension across the shoulders. Variable positioning, such as adjustable height desks and chair also can improve patient comfort.
Reference : Sheedy, J., Visual fatigue, Points de Vue, International Review of Ophthalmic Optics, N70
Source : www.pointsdevue.com
Points de Vue, International Review of Ophthalmic Optics, N71, Autumn 2014 Author : BOUVIER Luc

Annual exposure to solar radiation is three times higher in children than adults. Moreover, because of their physiology, children’s eyes are more vulnerable and require special protection against UV rays and blue-violet light. Designed for children as well as adults, the new Crizal® Prevencia® lenses are completely transparent, providing optimal photo-protection from day to day. The use of sunglasses will ensure additional protection in direct sunlight.
The harmful effects of chronic exposure to ultraviolet radiation and the blue-violet component of visible light are now clearly established as factors in the development of ocular diseases such as cataracts and AMD. [1] The cumulative effect of this exposure over a lifetime contributes to accelerating onset of these serious conditions. And this process begins in early childhood: children are doubly exposed to the risks posed by these harmful light rays.
The primary risk factor in children: overexposure
To begin with, children spend three times longer outdoors than adults, which increases their exposure to the most powerful source of UV rays and blue light: the sun. LED screens (tablets, smartphones, computers, etc.), which are new sources of blue-violet light, intensify this exposure further, as children come to use them more and more frequently and at a significantly earlier age. In the UK, the use of tablets at home among children aged 5 to 15 trebled between 2012 and 2013 (from 14% to 42%). One quarter (28%) of three- and four-year-olds use a tablet at home. [2] Nearly 20% of French children aged 7 to 12 were using a tablet in 2013 [3] – a figure three times higher than in 2012 (Fig 1).
All these devices are undeniably tools for enhancing cognitive development, improving awareness and teaching children to master the digital world. However, they can also foster an addiction to virtual environments and lead to difficulty sleeping. Their use needs to be limited and supervised through parental controls on the content and length of each child’s daily use. The growing number of screens that are backlit with cool white LEDs, which are known to generate potentially harmful blue-violet light, may increase the risk of chronic photo-toxicity over time.An additional risk factor in children: the permeability of the visual system
In early childhood, the crystalline lens is much more permeable to harmful UV and blue-violet rays, a significant portion of which can reach the retina (Fig. 2). Retinal exposure to UV radiation may lead to rapid growth in the concentration of lipofuscin during the early years of life [4] (Fig. 3); lipofuscin can subsequently prove toxic to the retina when subjected to blue-violet light.
The importance of risk prevention and education
It seems appropriate, then, to create solutions for preventing this risk and protecting children from a very young age. The parallel with skin should serve as a warning: according to the WHO, excessive exposure to the sun in childhood can contribute to skin cancer later in life. [5] Although there is growing knowledge of the need to protect children’s skin from the sun, and a wider range of specially de-signed protective sun cream (SPF 50+) is available for their use, the same cannot be said for eye protection in children. Adults, however, protect themselves better than their children: a U.S. study showed that just 48.4% of the parents surveyed use sunglasses to protect their children’s eyes. [6] A separate study in France revealed that 84% of parents own at least one pair of sunglasses, compared to 68% for their children. [7] But even among children who have sunglasses, the nuisance of using them means they are worn far less often than circumstances require. You need merely visit the beach in summertime to discover that the number of children wearing sunglasses remains quite small.
Fig. 1: The spread of personal devices among children (ages 7 to 12) and teenagers (ages 13 to 19) in France (Source: Ipsos).
Fig.2: Total transmittance of clear ocular media of aging human eye. Fitted from the CIE 203:2012 data. Does not take into account cataract surgery beyond 60 years old.
Fig. 3: Rapid increase in lipofuscin concentration between the ages of 0 and 10.
Source: Adapted from (Wing et al., IOVS, 1978), ex vivo, in the total RPE. For the vivo, at fovea and 7° temporal to the fovea, see (Delori et al., IOVS, 2001), faster increase with age.Crizal® Prevencia® Kids: the solution offering everyday protection for children
For children who already wear corrective lenses, there are increasingly effective solutions for daily protection. Up until recently, the only consistent way to filter out both blue light and UV rays was to wear tinted filters (yellow, orange) inside and/or sun lenses outside. This solution already represents a significant burden for older patients; the idea that this approach could be used with children on a daily basis, purely for protection, is unthinkable.
Crizal® Prevencia® Kids is a daily-wear lens that is especially tailored to the needs of children.Moreover, these filters completely eliminate blue light, distorting our color perception and potentially depriving the eye of the benefits of the blue-turquoise component of the visible spectrum (465-495 nm), which regulates our biological clock and in particular our waking and sleeping phases. It was in response to this need for a simple and effective form of prevention that Crizal® Prevencia® lenses were designed, for use by both adults and children.
These antireflective lenses come with a new interferential filter that provides selective protection (Fig. 4). Harmful light rays are filtered so as to reduce the effects of UV rays and blue-violet light (415-455 nm) on the crystalline lens and retina. The blue light that is beneficial to our bodies is maintained. Crizal® Prevencia® allows 96% of blue-turquoise light to pass through. The lens offers guaranteed transparency, with transmission of more than 98% of visible light to ensure optimal vision.Proven in vitro effectiveness
Crizal® Prevencia® lenses mark the culmination of lengthy research conducted in cooperation with Paris Vision Institute (IDV), considered one of Europe’s premier integrated research centres specializing in eye disease. To demonstrate the efficacy of these lenses in protecting retinal cells, the IDV conducted an in vitro experiment which revealed that in retinal pigment epithelium cells protected from blue-violet light by Crizal® Prevencia®’s interferential filters, the rate of cell death through apoptosis fell by up to 25% in comparison with unprotected cells. [8]
The most visible proof of the protection offered by Crizal® Prevencia® lenses is the color of the residual reflection produced by its filter: blue-violet (Fig. 6). When the lenses are exposed to the harmful component of blue light, these rays are partially reflected, and that distinctive reflection – which can be shown to future wearers when they purchase the lens – is a reliable indicator that the eye is being protected.Certified UV protection (E-SPF ™ 25)
Children spend three times longer outdoors than adults, which increases their exposure to the most powerful source of UV rays and blue light: the sun.When it comes to protection against UV rays, Crizal® Prevencia® Kids lenses offer the same level of protection as all the other untinted lenses in the Crizal® range, certified by an E-SPF® (Eye-Sun Protection Factor) of 25. Coupled with the Airwear®material that prevents any UV light rays from passing through, Crizal® Prevencia® lenses include a filter on their inner surface that virtually eliminates UV reflection into the eye.
Fig.4 The selective protection offered by Crizal® Prevencia®. Harmful forms of light (UV, 400-450 nm blue-violet light) are filtered out, while the useful and beneficial portion of the spectrum is preserved virtually in its entirety.
Fig. 5: Comparative results between Crizal® Prevencia® and the naked eye of RPE cell death by apoptosis, exposed for 18 hours in vitro to normalized sunlight for a 40 year old human eye.This UV exposure from the rear of the lens can be quite significant, accounting for up to half of all UV exposure for unprotected eyes. [9] Before the introduction of the most recent Crizal® lenses, anti -reflective lenses on the market still reflected a substantial amount of UV radiation. [10] The E-SPF®index, developed by Essilor, is the only international rating that measures the protection offered by a given lens on both its outer surface (for light transmission) and its inner surface (for reflection back into the eye). A factor of 25, currently the highest on the market for an untinted lens, indicates that the eye receives 25 times greater protection than it would otherwise (the sun lens offers an E-SPF® of 50+). The E-SPF® index gives eye care professionals a standard they can use with children who wear lenses and their parents, who are already familiar with the SPF index used for sun creams.A lens designed for children
In order to provide greater overall protection against harmful light rays, Crizal® Prevencia® Kids is a daily-wear lens that is especially tailored to the needs of children. Its effective anti-reflective treatment ensures perfect transparency, which means vision quality and comfort, notably for classroom learning and when viewing a screen.
In early childhood, the crystalline lens is much more permeable to harmful UV and blue-violet rays, a significant portion of which can reach the retina.When used with Airwear® material, Crizal®Prevencia® lenses are the most shock-resistant on the market, 12 times more resistant than standard lenses – which will reassure parents of even the most daredevil children. They also have the advantage of being 30% lighter and 20% slimmer to suit fragile noses, so children are more likely to accept them. In addition, Crizal® Prevencia® lenses are treated to provide maximum resistance to two things much feared by parents: scratches and smudges. These lenses – easier to clean than any other lens on the market – are ideal for children whose lenses quickly become dirty.
Fig. 7: The selective light protection of Crizal® Prevencia® on front and back sides.
Conclusion
We need to take steps as early as possible to protect eye health and prevent the risks posed by the harmful effects of UV rays and blue-violet light, because young children are especially vulnerable to the damage they can do. All children’s eyes need to be protected in the outdoors with appropriate equipment that provides good coverage against the sun when sunlight is strongest, alongside the proper precautions for protecting their skin: sun cream, a wide-brimmed hat, avoiding exposure when the sun is at its most intense.
For children who wear eyeglasses at all times, vision health professionals can now recommend the kids’ version of Crizal® Prevencia® lenses.
AWARDS / HONORS / SUCCESSES Crizal® Prevencia® lenses received numerous honors worldwide in 2014:
• in Canada: they were voted “Product of the Year” (the most innovative product of 2014 in the Optics category) by a panel of experts and consumers
• in France: Essilor’s R&D team accepted an award for technological innovation – the “Prix Fibre Innovation 2014” – given to Crizal® Prevencia® lenses at a daylong event hosted by Opticsvalley, an optics trade group, at the Université Pierre-et- Marie-Curie in Paris
• in Australia: the entire range of Crizal® UV treatments won certification from Cancer Council Australia, an organization that is unmatched worldwide for its experience in preventing risks from UV radiation. This is the first seal of approval of its kind for an interferential treatment in the history of ophthalmic optics.
CORRECTIVE LENSES TO PROTECT AGAINST UV CORRECTIVE SUN LENSES For everyday protection against the cumulative effects of exposure to UV rays, lenses with protection factor E-SPF ™ 25 offer the highest level of protection available for clear lenses. Crizal® lenses were the first in this category to offer this level of protection. They are available in an extensive range for all wearers, both children and adults (Crizal® Kids UV, Crizal®Prevencia®, Crizal Forte® UV, Crizal® Alizé® UV, Crizal Easy®UV). Associated with materials that absorb UV, Crizal® lenses benefit from technology that considerably reduces the eye’s exposure to UV due to reflection from the inner side of the lens. For optimal protection from the sun, Crizal Sun® UV lenses have protection factor E-SPF ™ 50+. They offer the essential level of protection when conditions demand the wearing of sun lenses (strong sunlight altitude, beach, etc). Crizal Sun® UV can be associated with tinted lenses or Xperio®polarizing lenses.
Points de Vue, International Review of Ophthalmic Optics, N71, Autumn 2014
Author : BOUVIER Luc
Source : Points de vue Points de Vue, International Review of Ophthalmic Optics, N72, Autumn 2015
What effects do digital displays have on health? The main risks, whether they are known, suspected or potential, primarily concern vision, but may also affect other functions. Experts are reassuring however: good visual hygiene, regular eye exams by professionals, appropriate optical solutions and enhanced public awareness provide effective prevention.

“Our visual system is biologically designed for distance vision. Near vision is only an accommodation reflex that helps us quickly identify objects close at hand. Our eyes are not designed to stare at screens for hours on end.” José de Jesús Espinosa Galaviz

“A reduction in the frequency of blinking during screen use increases the severity of such symptoms as dry eye or irritation and blurred vision. Smartphone users tend to hold their phones very close to the face, thus requiring an intense accommodative effort causing eye strain or headaches.” Sebastian Marx
“In such rapidly developing cities as Singapore, we see concomitant growth in the number of people working in offices and cases of asthenopia, sensitivity to light, transient diplopia and so on.” Koh Liang Hwee
“The increase in ophthalmic disorders is linked to the proliferation of screens and the time spent watching them: in the classroom (from primary school to postgraduate courses, including tablets, computers, electronic tables, etc.), but also at all ages via the social networks, television and e-books, which are becoming increasingly popular.” Helen Summers
“No clinical study to date has demonstrated that overexposure to digital displays is the cause of early macular degeneration. However, blue light emissions are a reality and over time we are bound to see a clinical impact. Concerning the increase in cases of myopia, various studies point to the possible influence of digital displays used at ever closer distances. We still need to understand why certain subjects develop myopia and others don’t, even among twins.” Sebastian Marx
“The main risk for the younger generation is myopia, perhaps not true myopia, but rather an ‘accommodative spasm’ (i.e. near point stress according to Skeffington), since the human eye and brain were not designed for extended near vision.” Aravind Srinivasan

“In the medium and long term, digital displays affect people in different ways. The impact is not solely ophthalmic. The symptoms are varied, suggesting both physical disorders (neck and back pain, etc.) and psychological disorders (fatigue, irritability, poor concentration, memory problems and so on).” Aravind Srinivasan
“Overexposure to blue light emitted by screens can disrupt the secretion of melatonin and thus affect the quality of sleep. Eye strain can also have an effect on productivity and lead to other disorders, such as stress, anxiety or mood swings.” Koh Liang Hwee
“Ever more pervasive video gaming is associated with player immersion and strong screen flicker. These two situations can eventually stimulate systemic and endocrine functions, resulting in elevated cortisol levels. The main repercussions have been found to affect sleep, behavior, mood, motivation and learning.” Helen Summers
“Consumer awareness campaigns are an important means of highlighting the risks and symptoms related to digital displays and offer an opportunity to stress the need for regular eye exams.” Aravind Srinivasan

“Every person consulting a vision care professional should be informed of the impact of digital devices and blue light, as well as the importance of good visual hygiene and the availability of optical solutions. A wide range of high-quality solutions are available; it is regrettable, however, that current prices limit their use primarily to adults rather than children.” Helen Summers
“A new specialty, ergo-optometry, could be created. The ergo-optometrist would counsel patients on how to take better care of their visual health,explain what products to use to treat dry eye and provide personalized information with regard to lenses and frames, even for patients without refractive error. Overweight people can contact Weight Watchers. People with ophthalmic problems should be able to contact Eyes Watchers.” Joachim Köhler
“We are not usually aware of our posture; our organism chooses the most appropriate position for a given situation, without worrying about potential physiological repercussions. It is essential to adopt good posture. For reading, I recommend the Harmon distance at a minimum; this is the distance from the tip of the elbow to the middle of the index finger.” José de Jesús Espinosa Galaviz
“Good visual hygiene also includes: an ergonomic work space; good posture, a straight head and back; good lighting, with lower lighting for screens and adequate room lighting; breaks every 20 minutes; alternating between near and far screen distances, and suitable ophthalmic lenses.” Helen Summers
How are digital devices influencing the everyday lives of vision care professionals? New consultation protocols, near vision refraction and control methods appropriate to digital displays, personalized counseling and more frequent continuing education are the main developments cited by experts. Many professionals are incorporating digital tools into their practices to better assess users’ needs. In the context of overexposure to digital devices, experts are also beginning to take more interest in children and emmetropic people (without refractive error).
“Just a few years ago, protocols were established on the basis of the symptoms one should look for rather than on patients’ needs depending on their environment. This approach is now changing.Currently, in addition to patients’ histories, we are also interested in their concerns, expectations, environment and so on, and we are adapting protocols accordingly.” Luis Ángel Merino Rojo
“For people who rely heavily on their near vision, I apply a protocol based on behavioral optometry. This approach is important when prescribing the best lenses for a particular type of activity.” José de Jesús Espinosa Galaviz
“My approach? First I exclude ocular pathology and perform a refraction. Then I evaluate the patient’s visual faculties (accommodation, convergence, ocular mobility and sensory aspects such stereoscopic vision, etc.). Once all these criteria have been evaluated, the treatment strategy can be defined.” Elizabeth Casillas
“Far vision refraction is often performed using cyclopegic eye drops with a refractometer. Near vision is examined with trial frames equipped with interchangeable lenses to better evaluate posture, head position and reading distance in relation to a support, computer or digital device. Instruments such as ‘Capture I’ or ‘Visioffice®’ are used to measure frame parameters and such individual parameters as pupillary distance and the eye’s center of rotation.” Helen Summers
“My staff has slightly modified their refraction methods to adapt to digital technologies. We placed a smartphone and tablet in the consulting room and, after the examination, we ask patients to read what is written on the screen. If they are unable to do so, we orient them towards specific lenses. Otherwise, all is well! By using digital devices to test near vision, we fit in more closely with our patients’ digital lifestyles.” Joachim Köhler

“There are several complementary approaches. The first involves optical correction, with hightech lenses offering optimal vision quality and protection. The second approach involves training, consisting of various exercises designed to improve visual capabilities. The third approach involves education in visual hygiene (posture, breaks, a good work environment, etc.). The final prescription depends on the age and issues of each patient.” Elizabeth Casillas

“The patient’s age affects the proposed treatment. People with presbyopia will be advised to wear progressive lenses, with a coating (i.e. a filter) suited to the specific issues posed by digital devices. For younger children, with or without a correction, lenses must primarily meet the objective of protecting their vision against the harmful effects of screens.” Aravind Srinivasan
“People working on computers are advised to have regular exams, in order to identify any symptoms of ophthalmic stress. The prevention aspect is particularly stressed for children, especially for children under 10.” Helen Summers
“We must be attentive to each of our prescriptions, always follow the same consultation protocol, compare feedback from each patient and keep a record of all results.” Berenice Velázquez
“Information provided by researchers, universities, specialized societies, suppliers and the like, helps us stay on top of new developments and provide increasingly personalized solutions. We must make an effort to step out of the ‘comfort zone’ of standardized options and adapt them to individual needs.” Sebastian Marx
“We have a real role to play in the treatment of disorders related to digital displays and must devote more time to informing and educating ourselves and to testing new solutions. In this regard, it could be useful to reinforce the sharing of experiences and dissemination of information through forums and professional networks.” Elizabeth Casillas
“My colleagues and I feel that emmetropes (i.e. people without refractive error) have been completely forgotten by our profession. During screen use, they are exposed to the same risks as glasses wearers. So it is important to educate them about the existence of simple solutions and practices to fight against asthenopia and other disorders related to digital devices.” Luis Ángel Merino Rojo
“It would be useful to mount a major information campaign on the risks of overexposure to digital displays. And explain that vision care professionals have solutions to respond to these issues, even for emmetropes.” Berenice Velázquez

“For vision care professionals, digital technologies make it possible to share cases and experience, to the benefit of patients.” Jaime Bernal Escalante
“Digital tools and certain applications can be used to take a number of different measurements: asthenopia, the quantity of blue light emitted by screens, etc. They can also be used to disseminate recommendations aimed at optimizing visual comfort and participate in the therapeutic education of users.” Berenice Velázquez

“There is a paradox. On the one hand, we have more and more technological tools available to us (auto-refractometers, digital phoropters, photo and video sharing capability to improve diagnosis, etc.), but on the other hand, we have a new generation of professionals who no longer know how to perform an exam without these devices. The right balance must be found between the assimilation of new technologies and basic knowledge.” José de Jesús Espinosa Galaviz
How do we anticipate future issues and respond to the realities of a multi-screen world? Between increased research efforts and the development of technological innovations that will facilitate customized products and services, the various ideas outlined offer a glimpse of the future of the ophthalmic optics sector, which is in a position to turn the digital challenge into a real growth engine.
“Technological progress is making rapid headway, but the ophthalmic optics industry should be further ahead than it is if it is to adequately meet the health challenges associated with digital displays. It is important to invest more in health research in general and vision health in particular.” José de Jesús Espinosa Galaviz
“New studies on the relationship between blue light and macular degeneration and the connection between the development of myopia and digital displays could provide clinical responses to current hypotheses based solely on interpretation.” Sebastian Marx
“We must continue research efforts on myopia and its development, solutions to amblyopia, eye reactions during screen use, night vision, light radiation, etc.” Luis Ángel Merino Rojo

“All studies focusing on the exact relationship between connected life and ophthalmic disorders should prove useful. And in my opinion, the development of shared databases would be a real ‘plus’ for all vision health players.” Jaime Bernal Escalante

“More precise measuring equipment. The fact of having 20/20 (10/10) vision reveals nothing about the way patients’ use their eyes while watching a screen.” Elizabeth Casillas
“Tools to measure the impact of luminous digital displays on the eye.” Aravind Srinivasan
“New products, particularly ophthalmic lenses capable of protecting the eyes against technological ‘radiation’.” Jaime Bernal Escalante
“The ideal lens: a product capable of integrating all treatments and filters on demand, based on the individual needs of each patient.” Koh Liang Hwee
“A completely innovative approach, with ‘flexible’ smart lenses capable of adapting their optical properties to specific situations. A high level of modularity that could involve the use of electronic components.” Sebastian Marx

“The multi-screen environment is part of daily life. This environment can potentially pose certain risks, particularly for the eyes, and it is up to us as vision care professionals to concern ourselves with these risks and provide some answers, either directly or via the Internet. Indeed, technological and societal developments are opening up new fields of practice that offer our industry an opportunity to evolve! Personally, however, I prefer direct contact with patients, to show them that I am indispensable as a specialist.” Joachim Köhler
“New visual needs concern a large number of everyday activities; therefore growth opportunities for the vision health sector can only increase. The solutions developed must provide added value: filters to prevent eye strain or blue light-related risks, lenses capable of stimulating peripheral areas of the retina to fight against myopia or stimulate amblyopic eyes and improve their performance. There are still many little exploited or untapped areas that will undoubtedly drive development in the future. The response to digital issues is part of this.” Luis Ángel Merino Rojo
The new digital era is witnessing new societal, sensorial and behavioral transformations. This brief survey of the situation worldwide highlights the increased overall level of awareness of the ophthalmic optics sector confronted with the rapid, wide-scale changes driven by the emergence of digital technology and, more particularly, its impact on users’ vision and posture. From stronger prevention efforts to personalized treatment options, without forgetting projections for the future, the vision health sector is joining forces to adapt to developments, anticipate upcoming challenges and provide better performing solutions for ametropic and emmetropic patients of all ages.
Insights collected by Oliver Vachey, science journalist.
Source : Points de vue
Source : Points de vue
Each day, our retina absorbs millions of billions of photons with an expected increased magnitude due to our new light exposure behaviors. Day after day, these streams of photons can induce irre-versible eye damage and contribute to the onset or development of debilitating eye diseases. The phenomenon is aggravated by the accelerated ageing of the world population, since an ageing eye is more photosensitive along with altered defense.
A better understanding of the pathogenesis of vision-threatening diseases, a sharp analysis of light/eye interactions, and an individual risk profiling for these eye conditions are now urgent to provide appropriate and personalized eye photo-protection solutions, starting with eyewear, for efficient and long-term prevention.
While light is necessary and beneficial to visual and non-visual functions, any optical radiation might potentially be hazardous to the eye if it is received and absorbed by eye tissues at doses capable of causing photomechanical, photothermal or photochemical reactions. On the one hand, brief and extreme bright light exposure may induce mechanical or thermal permanent and rapid eye injuries. On the other hand, moderate light exposure for an extended period of time may result in progressive biochemical changes and ultimately induce irreversible cell death. For this chronic lifelong eye light damage, the spectral specificity of light is critical. In particular, UV radiations and high-energy visible light are pointed out as high risk spectral bands respectively for the anterior eye and the retina.

Chronic eye exposure to solar UV radiations has been progressively associated with the pathogenesis of numerous cornea and crystalline lens diseases. If additional photobiology studies would be of interest to better dissect the intricate link between UV and the eye, sufficient in vitro, in vivo and epidemiology data confirm the contributory role of UV in numerous diseases of the anterior eye, such as cataracts, pterygium, conjunctivitis, pinguecula, climatic droplet keratopathy, ocular surface squamous neoplasia, etc. (for more details, see Points de Vue no. 67 [1] ).
In 1956, Kerkenezov observed an early clinical indication of the role of UV in pterygium. [2] Later, Minas Coroneo evidenced that peripheral light focusing by the anterior eye to the sites of usual locations of pterygium and cataract is involved in the pathogenesis of these eye conditions. [3] The Chesapeake Bay study reported a significant correlation between the spatial zone affected by the climatic droplet keratopathy and the average annual UV exposure. Corinne Dot et al. evidenced that mountain professionals are at higher risk for cataracts. The POLA, Beaver Dam Eye and Chesapeake Bay epidemiology studies revealed a higher prevalence of cortical cataracts in populations living in bright sunny plains.
Public awareness has rapidly been high on the UV eye hazard since skin UV protection has now long been encouraged and normalized (SPF factors).
Since UV radiations are totally absorbed by the cornea and the crystalline lens after the age of 20, the most energetic light reaching the retina is blue light.
Photobiology studies on blue-light eye damage started half a century ago, with the landmark paper of Noell evidencing blue retinal phototoxicity in rodents exposed to white fluorescent lamps. [4] In 1972, Marshall, Mellerio and Palmer observed blue light damage in the pigeon cones. [5] Since then, with the advent of lasers, the number of photobiology studies on blue light has soared. Ophthalmologists themselves have been encouraging such phototoxicity and exposure threshold studies for their patients’ exposure when conducting laser surgery (for retinal procedures, also for refractive surgery) or for themselves considering the light intensity of ophthalmic instruments (slit-lamp and others). More recently, in the 1990s, the IOL industry has funded phototoxicity research to support the benefits and safety of the blue-light filtering IOLs implanted during cataract procedures.
In vivo experiments revealed that photochemical damages to the retina exhibit lower dose thresholds in the blue range compared to green and red [6] as evidenced in monkeys [7, 8], rats [9, 10, 11] and rabbits [12, 13, 14, 15, 16].
Blue light hazards were further studied on the outer retina (photoreceptors and retinal pigment epithelium (RPE) (Fig. 1), on immortalized RPE cells loaded with either oxidized photoreceptor outer segment [17], purified lipofuscin[18] or synthesized A2E [19, 20, 21, 22, 23] . A greater toxicity of blue light was demonstrated by exposing human RPE loaded with lipofuscin during 48 hours upon violet-blue-green light (390 nm – 550 nm, 2.8 mW/cm²) and yellow-red light (550 nm – 800 nm, 2.8 mW/cm²).[18] This cell death was mediated by apoptotic processes involving caspase-3 and p-53 activation.
Many of these studies suffer limitations such as not being precise enough on the light dose sent, or illuminating with very high irradiances that trigger acute light-toxicity mechanisms rather than lifelong cumulative exposure damage. Moderate irradiances and longer exposure should be sought when studying the pathogenic mechanisms of Age-Related Macular Degeneration (AMD) or diabetic reti-nopathy. Under the supervision of Professor Sahel and Dr. Picaud, Paris Vision Institute and Essilor researchers joined skills to go a step further from a photometry standpoint. By developing innovative cell illumination protocols and systems, we together have studied various phototoxic action spectra involved in the pathogenesis of severe vision-threatening diseases (AMD, retinitis pigmentosa, glaucoma, etc.). For instance, we have evidenced the precise phototoxic action spectrum of RPE within the blue-green range in sunlight physiological retinal exposure on an established in vitro model of AMD. [24] The 415 nm – 455 nm narrow spectral range was highlighted as the greatest phototoxic risk to RPE cells (Fig. 2).
In vitro and in vivo studies have progressively revealed a strong scientific rationale for cumulative blue toxicity on the outer retina. The understanding of cell mechanisms involved has provided crucial inputs on the pathogenesis of outer retina diseases, in particular AMD. First, cumulative exposure to blue light favors the accumulation of all-trans-retinal in the photoreceptor outer segments (POS). All-trans-retinal interacts with blue-violet light with a decreasing profile between 400 nm and 450 nm. Its blue photo-activation induces oxidative stress within the POS. This stress is normally compensated by retinal antioxidants and enzymes, but age progressively reduces anti-oxidative defenses, thus failing to compensate for the oxidative stress. The POS progressively oxidize, and their renewal into the RPE is more challenging as their membrane components are difficult for the RPE to break down. Thus, intracellular digestion is incomplete and generates an accumulation of residual lipofuscin in the RPE. [25] Lipofuscin is sensitive to blue-violet light. Blue photoactivation may generate reactive oxygen species. When the number of these species exceeds cellular defence capacity, RPE cells die by apoptosis. Deprived of these support cells, the photoreceptors deteriorate in turn, contributing to the loss of vision diagnosed in patients suffering from AMD. Age and light-related accumulation of lipofuscin in the RPE are major pathogenesis features of AMD.

Fig. 1: Retinal tissues Paris Vision Institute images by confocal microscopy. RGC=Retinal Ganglion Cells; IPL=Inner Plexiform Layer; INL=Inner Nuclear Layer; OPL=Outer Plexiform Layer; ONL=Outer Nuclear Layer; RPE=Retinal Pigment Epithelium
Numerous epidemiology studies confirm the correlation between blue light exposure and AMD. [26, 27, 28, 29, 30, 31, 32] The EUREYE study found significant association between blue light exposure and neovascular AMD in individuals having the lowest antioxidant level. In the Chesapeake Bay study performed on 838 watermen, AMD patients – compared with age-matched controls – were significantly higher exposed to blue over the preceding 20 years but equally exposed to UV, suggesting that blue light exposure is related to AMD. The Beaver Dam Eye Study reported a correlation between sunlight and 5-year incidence of early AMD changes. Leisure time spent outdoors while persons were teenagers (13 – 19 years) and in their 30s (30 – 39 years) was significantly associated with the risk of early age-related macular changes. A recent meta-analysis led by Sui et al. interestingly concluded that light is a risk factor for AMD. [33]
Beyond the outer retina, photobiologists have recently suspected that high energy visible light could also affect inner layers of retina, such as retinal ganglion cells (RGC). Specific blue light may be absorbed by chromophores located in mitochondria. As an abundance of mitochondria are localized in RGC and these cells are involved in the degenerative processes of glaucoma, we suspect that blue light is a risk factor for glaucoma as well. In ageing retina where functional mitochondria are no longer in an optimum homeostatic state [34] , blue light might dramatically precipitate the onset of glaucoma and other optic neuropathies. It could even contribute to accelerating glaucoma once diagnosed. [35, 36]

Light is suspected of being a risk factor in many debilitating eye diseases. For cataract and AMD, it is now well established: UV radiations accelerate the cataract onset while in AMD, blue-violet light exposure is a precipitating factor. For other diseases such as diabetic retinopathy or glaucoma, photobiologists suspect cumulative lifetime exposure to blue light contributes to the oxidative stress of specific retinal cells. In all cases, the contribution of light among other pathogenic factors grows with age and when the defence and repair mechanisms against photochemical damage are less effective, which is the case when the eye disease is already diagnosed (e.g. antioxidant enzymes such as SOD-2 or catalase are less effective).
European and ISO standards for sunglasses (EN 1836 and ISO 12312-1) and for tinted ophthalmic lenses (ISO 8980-3) have, for many years, used a relative spectral effectiveness weighting function S(λ) to characterize UV hazards. This was originally published in ICNIRP guidelines 1989 and is derived from an action spectrum for skin erythema.
A sister function in the blue range was proposed later, B(λ), derived from the seminal work by Ham et al. for the acute hazard on aphakic monkey eyes. B(λ) was defined by multiplying the spectral values of Ham et al.’s research with the spectral transmittance of the human lens. Nevertheless, there is no standard on cumulative blue light toxicity. New identification of phototoxic action spectra [24] should be advantageously used to create and/ or revise normative data on phototoxicity.
In vitro phototoxicity studies bring valuable and robust information on the light action spectrum as well as on the light-associated specific cell and disease biomarkers. The Essilor and Paris Vision Institute study with 10 nm step illuminations is a good illustration. Animal models (in vivo) are interesting living and integrative models of a disease. They make it possible to study the role of a specific gene (transgenic knock-out animals), the involvement of a specific toxicity pathway (e.g. inflammation, oxidative stress) or a disease target. They are essential in correlating disease with imaging, biology testing, immunohistochemistry or behaviour. But they have limitations: pathogenic mechanisms can differ from humans (e.g. using rodents in AMD models while rodents do not have a macula); light illumination is more intense and of a shorter duration than in real life [6] . While in vitro and in vivo experiments raise the understanding of a disease-specific light action spectrum and pathogenic mechanisms, the only clinical evidence of light-associated eye diseases is brought by longitudinal epidemiological studies. It is therefore necessary, when studying real-life eye chronic phototoxicity, to find light-specific markers of disease early-signs and disease progression.
Now that cataract is being better treated in eastern and southern countries, AMD, glaucoma and diabetic retinopathy are becoming the three major vision-threatening diseases. This threat is largely worsened by the world ageing and by lifestyle risk factors such as poor antioxidant diet. From a public health perspective, considering that it is now urgent to optimize the disease management for these three eye conditions, all efforts should be paid to raise awareness in the general population, especially in the sub-populations considered more at risk, to develop and select the most relevant tests for early diagnosis and disease profiling, ultimately to monitor the disease progress and to apply the most appropriate therapeutic strategy. This sounds obvious but we believe we have now reached a more mature stage of understanding of the disease mechanisms associated with the availability of a set of new diagnostic tools including eye imaging, biological testing of biomarkers and psychophysical methods. In order to manage effectively a multifactorial disease, there is an utmost need of disease profiling and monitoring and if applicable of multi-facetted therapy – aiming at multiple mechanistic targets – as well as prevention.
Individual risk profiling is defined by multiple intricate factors, source-, patient-and environment-dependent (Fig. 3).
Our own light exposure profile is defined by the correlation of the number of light sources, their local-ization, their spatial distribution, their radiance including directivity, but, critically, also their spectral distribution, the exposure duration and repetitions.
There is no doubt that solar radiations are the most harmful ones, since sun radiance is more than 100 times higher than the radiance of standard artificial lighting [37] and since daylight is rich in UV and blue light. The physical environment (ground reflectances, altitude, latitude, etc.) significantly modifies the amount of light received by the eye. The eye UV dose increases by 10% every 1,000 metres. While sand reflects 10% of UVB, water reflects 20% and snow more than 80%. Therefore, populations exposed to bright sunlight in high ground reflectance environments (mountain professionals, etc.) or living in very sunny plains are at higher risk of UV and blue-light related eye damage, including cataracts and AMD.

In addition to daylight, in our ageing and connected digital world illuminated by new solid-state lighting, our light exposure profile is rapidly and dramatically evolving. Starting at increasingly younger ages of our existence, our eyes are subjected to longer and simultaneous exposures, at shorter distances, with higher radiance and higher energy than with former incandescent sources.
Since they produce light with much lower energy consumption, these new solid state lighting sources have become the dominant domestic lighting technology. In Europe, by 2016, no traditional incandescent light sources will be available. The European lighting industry estimates that over 90% of the total luminaires world market will be based on solid state lighting products by 2020. [38] Beyond domestic lighting, the LED compactness plus the wide spectral range they can cover (monochromatic LEDs) have generated many new lighting applications, for mobile phone and tablet back lighting or even for toys and clothes.
New LED-based light sources may emit more blue than former incandescent lamps. [39] Current white LEDs are combining a blue pumped LED with a phosphor emitting at higher wavelengths. For mass production of white LEDs, blue diodes based on InGaN or GaN crystals are combined with a yellow phosphor (YAG:Ce or similar); they produce “cold-white” with a color temperature CCT equal or higher than 5500 K [39] . They may emit up to 35% of blue light within the visible range, much more than incandescent lamps (< 5%). To produce “warm-white” with a CCT <3200 K, with less than 10% of blue, an extra layer of phosphor emitting red light is needed, which significantly reduces the luminous efficacy of the LED.
At retinal level, received irradiance is directly proportional to the radiance of the light source. By having a small light emission area, LEDs have a higher radiance, which makes them brighter, even for the same irradiance level.
Worldwide initiatives have been launched to conduct a health risk assessment on systems using LEDs. A task group was for instance mandated by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) in 2008. They concluded that a photochemical blue light risk could exist, consecutive to prolonged white LED exposure. Risky light exposure profiles may be identified and related to high-risk populations (for more details, see Points de Vue no. 68 [40]):
– the daily adjustment and testing of high power cold white LEDs, by lighting installers, operators in lighting manufacturing facilities, show technicians and collectors, dentists, surgeons, etc.;
– the use of toys with LEDs, since children have a crystalline lens more transparent to blue light;
– automotive LED daytime running lights, when activated near children or photosensitive persons (aphake, pseudo-aphake eyes, people suffering from ocular photosensitive pathology or using photosensitive drugs, etc.);
– some directional LED lamps sold for home applications, if viewed at distances equal or shorter than 200 mm;
– the prolonged and repeated use of cold white LED-based devices by children and teenagers, especially in the evening, etc.

Fig. 3: Individual risk profiling is defined by multiple intricate factors.
Each person is unique. We do not respond to equal light exposure the same way. Genetics, morphology, ethnics, gender, age, behaviors (smoking, diet, etc.), squinting effects, eye protection (eyewear, shadow cap, nutraceuticals, etc.) are all contributors to a distinct personal risk profile.
Age, for instance, is largely involved in the progressive deterioration of visual functions such as dark adaptation. [41, 42, 43] These findings are supported by the histological observations that rods degenerate early in both ageing and AMD. [44, 45] With age, the number of photosensitizers is rapidly increasing in the retina, particularly in the RPE where the lipofuscin age pigment builds up. This increase is partly due to blue photo-ageing processes. Age plus cumulative blue light exposure may irreversibly alter the classical visual cycle in the outer retina, progressively leading to AMD.
In the case of AMD, Erica Fletcher et al. have interestingly reviewed the new means of detecting the signs of early-stage disease. [46, 47] AMD has genetic and environmental risk factors. Genetic testing is now readily available using a combination of 16 genes to help predict the risk profile of an individual. Among the genes associated with an increased risk of developing AMD is complement factor H (CFH), the mutations of which contribute to explain immune dysregulation in AMD. Considering retinal imaging, Hogg et al. have recently highlighted that a particular form of drusens called reticular pseudo-drusen, at a subretinal level, is a risk-factor for progression to late-stage disease. [48] A research team in the Netherlands has developed a software to analyze color fundus photographs so as to quantify and characterize drusens and determine a risk assessment. Using fundus autofluorescence (FAF), the RPE cell dysfunction can be detected through the accumulation of lipofuscin. Some scientists report that certain patterns in FAF imaging can help predict the evolution of AMD towards the choroidal neovascularization (CNV) late-stage form. Psychophysical methods can also help define if a subject is at greater risk of AMD or, later, of progressing to late-stage disease. Among those methods, dark adaptation disruption is an early marker of the disease. Next, it would be helpful to monitor the light exposure in cohorts of patients and try to associate disease progression. Therefore, a comprehensive clinical assessment and biomarker testing, including questionnaires (family history, lifestyle, etc.), visual examination and specific psychophysical methods, imaging, genetic testing and light exposure profiling can define an individual profile risk and help detect and characterize AMD at an early stage as well as monitor disease progression.
For most eye diseases, an individual can undergo a complete assessment of his or her risk profile specifically related to a disease and then if needed, once informed and educated, decide to adopt a more thorough and frequent medical and self-surveillance so as to detect early enough the onset and progression of a disease and to take personalized prophylactic actions.

Light protection should be part of a personalized prophylactic program instructed by eye care practitioners. If a patient is profiled at risk of one of the vision-threatening diseases, and if the precise light spectrum incriminated as a risk factor is known (such as blue-violet light for AMD), then it is in the patient’s interest to protect himself selectively, especially when a portion of visible light needs to be filtered out as part of the preventative measure. Contrary to UV radiations, which can be fully blocked with no compromise on vision, filtering out visible light is always a trade-off with color vision and other physiological functions such as chronobiology or scotopic vision. Fortunately, the ophthalmic optics industry, in recent years, has fostered or benefited from the emergence of innovative narrow-band filtering technologies and has now started to develop photoselective and photoprotective ophthalmic filters. These new lenses offer practitioners an effective complementary tool to the armamentarium of prophylactic solutions available to their patients.
Major eye diseases, such as cataracts, AMD, glaucoma or diabetic retinopathy, have a tremendous impact on patients’ quality of life but also significant implications on the cost of healthcare. The threat is enhanced by the accelerated ageing of the world population and by evolving behavioral and environmental factors. Photobiology research has progressively identified light as a risk factor for these multifactorial conditions. Further photobiology studies, in vitro and in vivo, with well-calibrated light conditions, along with epidemiology studies with proper spectral light exposure quantification, are now necessary to identify significant correlations between light action spectra and pathogenic mechanisms. As initiated in RPE and RGC models, Essilor and the Paris Vision Institute are pursuing their efforts in developing accurate photometric tools (devices and protocols) for controlled cell illumination. A better understanding of the pathogenesis of eye conditions would interestingly be complemented by a better disease management, including real-life phototoxicity studies to find light-specific early markers of a disease and individual risk profiling. In our digital world with huge technical tools in image analysis and miniaturized sensors, the personalized and early follow-up of an eye disease is now made possible and paves the way for relevant personalized prevention. In the meantime, technology breakthroughs in the ophthalmic optics industry are meant to provide new effective solutions to design efficacious and personalized photo-protective and photo-selective eyewear.
U.S. optometrists begin global initiative of eye disease prevention – Points de Vue.
Macular degeneration (AMD) and cataracts play a major role in the United States health care system and global effort directed to the prevention of these conditions now is part of optometry initiatives. To benefit society both from a financial and a productivity perspective, optometrists focus on four areas in clinical practice: protective lenses, nutraceuticals, genetic testing and periodic examinations.
 Fig.1 : Demonstrating Crizal® Prevencia® to a patient
Fig.1 : Demonstrating Crizal® Prevencia® to a patient
In February 2014, approximately 24 optometrists gathered at the University of Houston for the first ever Ocular Surface Disease Wellness Conference. The subject of “wellness” and disease “prevention” were addressed in this historic two-day meeting. Prior to this meeting, most specialized gatherings by optometrists addressed disease diagnosis and treatment, rather than prevention. The concept of disease prevention is unique in the vision care arena. One possible exception is in the area of myopia where efforts to retard progression have been tried using bifocal eyeglasses, contact lenses (orthokeratology) and pharmacological agents (atropine).[1] A follow-up meeting has been scheduled for December, 2014 in Dallas, Texas.
Two conditions that lend themselves to prevention discussions by optometrists in the United States include ocular surface disease (OSD) and ocular damage from high-energy visible light (HEV) as well as damage from ultraviolet light (UV). Macular degeneration (AMD) and cataracts play a major role in the United States health care system and any effort directed to the prevention of these conditions will benefit society both from a financial as well as a productivity perspective. Cataract surgery is the most commonly performed surgical procedure in the United States today. The average cost of cataract surgery today is $3,230 per eye [2], and it is rising because of the use of new technology (laser cataract surgery and multifocal IOLs). Estimates of the global cost of visual impairment due to age-related macular degeneration is $343 billion.[3]
Specific to AMD prevention, U.S. optometrists now focus on four areas of preventative steps. Those areas include nutritional supplements, genetic testing, specialty lens coatings to block selective wavelengths of blue light, and periodic dilated fundus examinations with OCT studies. While there are several OCT models available, we have personally been pleased with our newer Cirrus™ HD-OCT instrument. Genetic risk assessment for age-related macular degeneration is becoming commonly employed in the United States for those patients with risk factors. Steve Arshinoff [4] writes: “Previously we considered the phenotypic appearance of the eye, macular pigment levels and patient-related non-genetic factors to determine AMD risk.” [4] Genotyping with commercially available genetic testing (Macula Risk™, RetnaGene™) now allows us to predict with 90 percent accuracy an individual’s 2-, 5- and 10-year risk for progression to advanced AMD. Following the reporting of the Age-Related Eye Disease Study 2 (AREDS2) (NEI) results, we now have definitive information on AMD prevention and progression using particular nutritional supplements, although further research is necessary.
Pathophysiology and economics
The number of people living with macular degeneration is similar to that of those who have been diagnosed with all types of invasive cancers.[5] As many as 11 million people in the United States have some form of age-related macular degeneration. The number is expected to double to nearly 22 million by 2050. Most researchers believe that blue light exposure has a role in the pathogenesis of AMD. According to Margrain et al.[6]: “Laboratory evidence has demonstrated that photochemical reactions in the oxygen-rich environment of the outer retina lead to the liberation of cytotoxic reactive oxygen species (ROS). These ROS cause oxidative stress, which is known to contribute to the development of AMD. The precise chromophore that may be involved in the pathogenesis of AMD is unclear but the age pigment lipofuscin is a likely candidate.”They continue: “Studies in human macular pigment density and the risk of AMD progression following cataract surgery lend further weight to the hypothesis that blue light exposure has a role in the pathogenesis of AMD but the epidemiological evidence is equivocal. Blue-violet light has a twofold effect on lipofuscin. It causes an increase in production and also activates its phototoxic components (free radicals), causing the death of RPE cells. On balance the evidence suggests but does not yet confirm that blue light is a risk factor in AMD.”[7] Research by the Schepens Eye Institute (Harvard University) suggests that a low density of macular pigment may also represent a risk factor for AMD by permitting greater blue light damage.
Science
Existing artificial light sources are basically of two types: incandescent (includes halogen) and luminescent (fluorescent and LED). Incandescent lights are becoming difficult to find in the typical home repair stores in the United States as the newer LED light sources begin replacing them. These newer light sources are much more energy efficient, have a longer lifetime and the government has decreed that this exchange takes place. It is thought that by 2020, 90% of all light sources worldwide will be based on solid state lighting products and LEDs. These newer light sources give off a greater proportion of blue light than the older incandescent bulbs. We know that the sun is the standard light source. The blue light proportion of our daylight in the entire visible spectrum varies between 25% and 30%. We know that blue light is vital to a number of physiological processes [8] and interfering with it may have adverse effects. A recent study by Gray and colleagues in the Journal of Cataract and Refractive Surgery found that patients with blue-light filtering IOLs performed significantly better under driving conditions with glare compared with similar patients who had clear IOLs. Dr. Henderson and her colleagues see no harm posed by blue filters, at least in visual parameters: they “feel that the potential protection against AMD is worth it.”[9]
Clinical practice
Several groups around the world have studied the potential health risks of products using LEDs. Basically three high-risk populations have been identified: (1) children and aphakes who receive a higher blue light proportion on the retina, (2) those individuals suffering from ocular photosensitive pathologies or using photosensitive drugs (light-sensitive agents used in photodynamic therapy such as Verteporfin used to ablate blood vessels in the eye when treating wet macular degeneration), and (3) those individuals who are daily exposed to LEDs while using short viewing distances.
As a practical matter, optometrists and ophthalmologists in the United States have begun the process of utilizing electronic medical records (ObamaCare). During the early part of the patients’ examination they are asked several questions by the technician and those individuals who fall into one of the above three groups are then counseled on the particular risks they face and are prescribed spectacle lens treatments which will help protect them from the increased threats offered by increased blue light presence. Because optometrists are the guardian of good vision, it is important for us to counsel patients about modifiable risk factors. Two of those risk factors include smoking and cumulative light exposure, especially UV and HEV blue light.
We have found at the Clayton Eye Center in Morrow, Georgia that the best results are achieved when the doctor himself/herself initiates the conversation about blue light protection in the xamination room and then the dispensing optician reinforces the message. We specifically prescribe the new Crizal®Prevencia® lens treatment in order to selectively filter out only the dangerous wavelengths while allowing the good wavelengths to pass through. We know that blue wavelengths are the most potent portion of the visible electromagnetic spectrum for circadian regulation. Because the timing and quantity of light and darkness both affect sleep, evening use of amber lenses to block blue light might affect sleep quality. We have found over the past several months that our patients appreciate the fact that we are protecting their eyes with these discussions and we feel that to not educate our patients would be a great disservice. We reference data from the Beaver Dam and Blue Mountain studies, which implicate blue light as a factor for age-related macular degeneration, particularly following cataract surgery. Our clinic performs more than 3,000 cataract surgeries a year and each post-op visit emphasizes the potential risk of blue light. Our IOLs are blue blocking for added protection. Our practice recently became involved with an accelerated program emphasizing doctor-directed dispensing. Each of our nine optometrists now prescribes various lens products and coatings to each patient when indicated and outlines the specific products on a specially designed form and reviews these products with the patient. The patient is then escorted to the optical department from the clinical area by a technician or the doctor and the form is presented to the dispensing optician. Products such as AR, Transitions®, digitally surfaced progressive designs (such as the Essilor S Series ™) and Crizal® Prevencia® coatings have increased substantially as a result of this new process.
Several lens manufacturers have become involved with blue blocking technology; however, so far only Essilor has designed a coating that blocks specific wavelengths. VSP’s Unity BlueTech lens, Hoya’s Recharge ™ , PFO’s iBlucoat ™ and Signet Armorlite’s BlueTech (Indoor and Outdoor) all block HEV (high energy visible light) and offer improved contrast sensitivity. However, they also block the blue-violet range, which has been demonstrated to be “good” light and necessary for other functions, including increased contrast sensitivity and mood regulation.
The Age-Related Eye Disease Study 2 (AREDS2) was a multi-center, randomized trial designed to assess the effects of oral supplementation of macular xanthophylls (lutein and zeaxanthin) and/or long-chain omega-3 fatty acids (docosahexaenoic acid) [DHA] and eicosapentaenoic acid [EPA]) on the progression to advanced age-related macular degeneration (AMD). While the results of the study left several questions unanswered, it also led the way to changes with respect to the prescribing of nutritional supplements for those patients with early macular degeneration and those at risk. Optometrists in the United States now routinely encourage their patients to take these nutraceutical supplements as a matter of procedure and this author suspects that this practice will become standard of care in only a matter of time. There are several commercial products on the market to choose from: Bausch and Lomb’s Preservision Eye Vitamin AREDS 2 Formula Soft Gels are probably the most commonly used. This particular product is beta-carotene free, which is a positive for current/former smokers. Another product that I have frequently used is Science Based Health’s Macula Protect Complete, which is also beta-carotene free. The study demonstrated that there was a 25% overall risk reduction of progression to exudative AMD. The role of macular pigment (MP) is also acknowledged and many optometrists now measure macular pigment and dose supplements accordingly. The U.S. diet is known to be low in lutein and zeaxanthin.[10] The third carotenoid, Meso-Zeaxanthin, is a key carotenoid in the macula and even lower in the U.S. diet. We know that smokers are at high risk for AMD.[11] In smokers and former smokers, betacarotene has been associated with an increased risk of lung cancer. [12, 13, 14]
Genetic testing has progressed in several areas of medicine over the past ten years. One area that has enjoyed the benefits of continuing research is AMD. We can now make a prognosis to within 90% accuracy of how a patient’s eye disease will progress.15 Several research projects have demonstrated that those patients subjected to testing have better outcomes than those without. At the 2013 American Society of Retina Specialists Annual Meeting, Dr. Peter Sonkin, a retina specialist from Tennessee Retina, presented the results from an analysis of the impact of genetic testing in their practice over a five-year period. The data revealed that patients who had Macula Risk testing and were subjected to a stratified surveillance schedule as well as a patient education program had better visual acuities on presentation compared to those patients without genetic testing. The November 2013 Issue of Ophthalmology highlighted an article titled “Prediction of Age-Related Macular Degeneration in the General Population – The Three Continent AMD Consortium”, which is a study evaluating AMD prognostics using three prospective population-based studies: the Rotterdam Study, the Beaver Dam Eye Study, and the Blue Mountain Eye Study. The non-genetic model which included age + sex + BMI + smoking + AMD status has a 78% predictive accuracy, while the genetic model, which included genetics with the above criteria, had an 82% predictive accuracy. Using all available information I have now come up with a formula for what the primary care optometrist should now do to prevent vision loss. This protocol is used by our doctors and many others and is a compendium of existing good practices.
Optometrists in the U.S. have embraced genetic testing for AMD much in the same way other physicians have embraced genetic testing for cancer and several other diseases. There are over 2,000 tests available. Screening embryos for disease is becoming more frequent.
In summary, AMD is on the rise. As individuals continue to live longer, the optometrist is going to diagnose increasingly larger numbers of cases. Prevention is a must. We now have several tools that will allow us to aid in our preventative efforts. Government-mandated lighting changes will expose us to larger doses of potentially harmful HEV blue light. Computer usage continues to be on the rise and these tools as well as electronic tablets, smart phones and other games used at closer near point distances will also increase our exposure. By prescribing spectacle lenses that can aid in filtering out the noxious wave lengths, we may be able to prevent many individuals from acquiring this dreadful disease down the road. By adding genetic testing and nutraceutical supplements to our armamentarium, we may be doing the world a tremendous favor. Our job is vision preservation, and this is one way to accomplish that task. To not implement the above protocol but rather take a “wait and see” approach may be doing your patients more harm than good. The Optometric Oath promoted by the American Optometric Association includes the following charges:
Read the article HERE